TB T-Spot test (mycobacteria mycobacterium tuberculosis mtb latent igra gamma interferon)
Microbiology
Notes
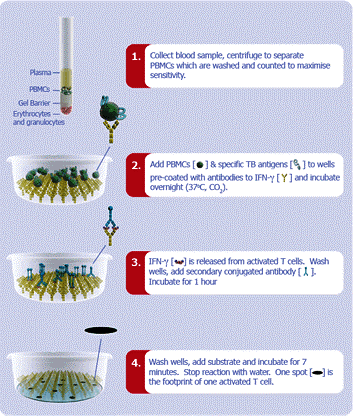
- T-SPOT TB is an in vitro diagnostic test for the detection of effector T cells that respond to stimulation by Mycobacterium tuberculosis antigens and is intended for use as an aid in the diagnosis of tuberculosis infection.
- The test is suitable for use with all patients at risk of latent tuberculosis infection or suspected of having TB disease, regardless of age, sex, ethnicity, therapy or immune status.
- The test should only be considered after discussion with a chest physician, Consultant Microbiologist or a specialist physician.
- Samples can only be taken Monday to Friday and must be received in the GRH Microbiology laboratory by 3.00pm. If taken in Cheltenham please allow time for transport to Gloucester. It is recommended that the request form is clearly marked as "URGENT" and that the sample is taken to CGH Pathology Reception by hand.
- Please inform the laboratory when the sample is being sent.
- See also:
Sample requirements
Blood in TWO 6 mL lithium heparin tube

For paediatric samples, use two paediatric lithium heparin collection tubes and collect as much sample as possible.
Required information
- Relevant clinical details
- Whether test is being performed to screen for suspected latency (asymptomatic) or for current, active TB infection
- M. tuberculosis exposure history
- Immunocompromise
- If test is being performed prior to immunosuppressive treatment
- If patient is a healthcare worker
- Please inform the laboratory when the sample is being sent
Storage/transport
Do not refrigerate or freeze whole blood samples
Store and transport at room temperature
Samples must be received in the GRH Microbiology laboratory by 3.00pm
Turnaround time
Sent to a National Reference Centre
2 - 3 days